Empowering health
About Sulfateq B.V.
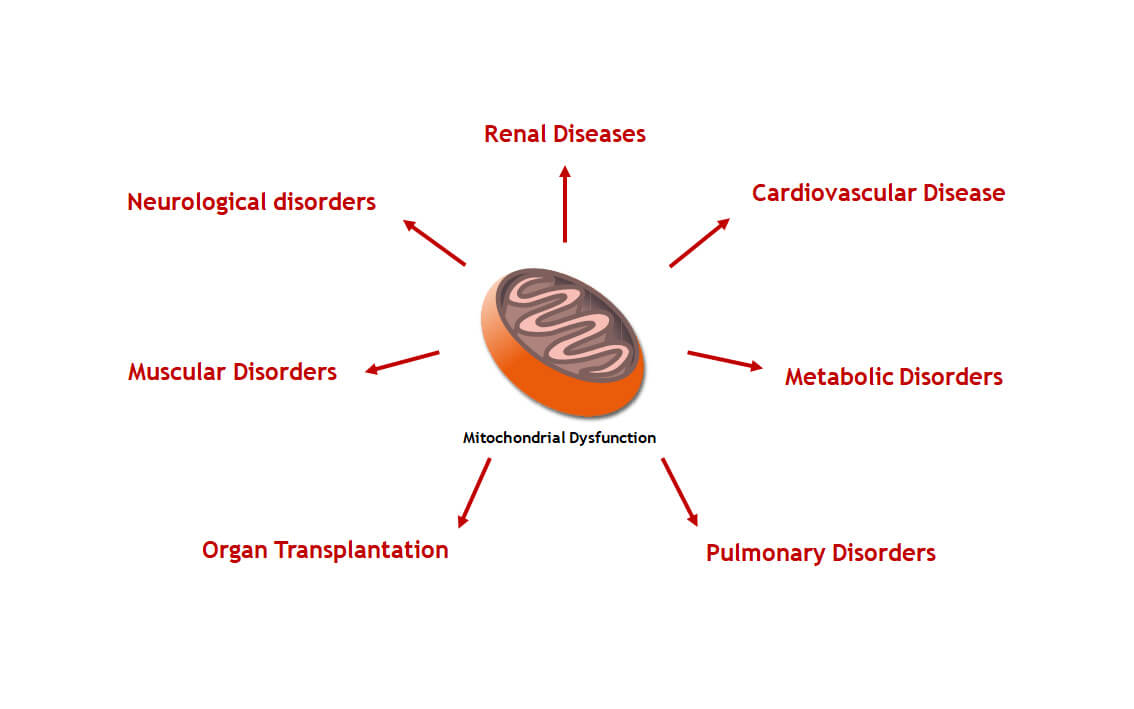
Sulfateq BV is an innovative privately-held biotechnology company that is developing breakthrough compounds for the treatment of a wide range of therapeutic indications related to mitochondrial dysfunction. Our compound library emerged from research on natures natural mechanisms to cope with mitochondrial stress, including hibernation. SUL compounds have an unique mechanism-of-action and the potential to revolutionize the standard-of-care of diseases associated with a declined mitochondrial function.
We strongly believe in the potential of our science to deliver new treatments and improve quality of life for patients with non-communicable diseases involving mitochondrial dysfunction.
We are always open for new innovative partnerships. Please contact us to discuss the opportunities.
GEN and Sulfateq shared the positive results from the multiple-dose Phase 1 study of SUL-238 at CTAD 2025
GEN Announces New Positive Phase 1 Trial Data of the Investigational Drug SUL-238 for Alzheimer’s and Other Neurodegenerative Diseases. Ankara, Türkiye, December 02, 2025 – GEN Pharmaceuticals (GENIL.IS), Türkiye’s leading specialty pharmaceutical company, has...
Sulfateq B.V.
+31 503137464
info@sulfateqbv.com
Admiraal de Ruyterlaan 5, 9726 GN, Groningen